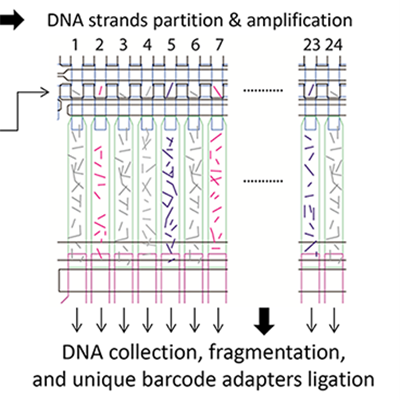
Potential applications include precise early cancer detection
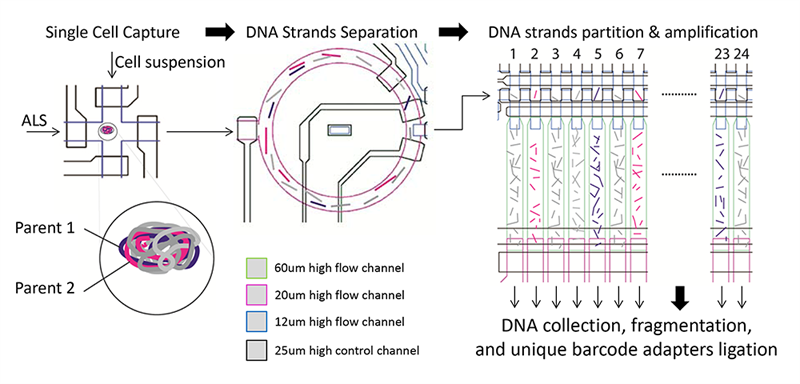
technology. Single cell in suspension identified by
imaging and captured, then chromosomal DNA
molecules are separated into single-stranded form.
Single-stranded DNA molecules are then randomly
distributed and partitioned in 24 chambers, then
pushed into an air-filled MDA chamber and reaction
solution. MDA reaction occurs after heat is applied to
the device at 30 degrees C overnight. Amplified
product in each chamber is collected and processed
into bar-coded sequencing library.
An interdisciplinary team of researchers at the University of California San Diego has developed a technology for very accurate sequencing and haplotyping of genomes from single human cells. Their findings were published online in advance of the Proceedings of the National Academy of Sciences (PNAS)* print edition.
“Accurate sequencing of single cells will enable the identification of mutations that cause cancer and genetic disease,” said senior author Kun Zhang, a professor of bioengineering in the UC San Diego Jacobs School of Engineering. “At the same time, precise haplotyping will allow for the genotyping of haplotypes, combinations of different genes or alleles as a group from either parent.”
Zhang’s co-authors from the Department of Bioengineering include professor Xiaohua Huang and postdoctoral researcher and alumnus Wai Keung Chu (M.S., Ph.D. ’11, ’16), who is first author on the PNAS article. Collaborators on the research from the Department of Computer Science and Engineering (CSE) include professor Vineet Bafna, who is a bioinformatics expert in the Center for Microbiome Innovation, the CHO Systems Biology Center and the Qualcomm Institute, all at UC San Diego. Others from CSE include Ph.D. student Peter Edge, and CSE alumnus Vikas Bansal (Ph.D. ’08), now a faculty-affiliate in CSE and professor of pediatrics in the UC San Diego School of Medicine. Adding to the project’s interdisciplinary roots is Department of Electrical and Computer Engineering (ECE) alumnus Ho Suk Lee (M.S., Ph.D. ’11, ’15), now at Broadcom, who lent his expertise in microfluidic devices for single-cell analysis as well as years of working in the bioengineering labs of both professors Zhang and Huang.

first author and postdoctoral scholar Eric
Wai Keung Chu (Bioengineering) and
Ph.D. student Peter Edge (CSE), who
works in a School of Medicine lab.
Clinical applications of genome sequencing demand high accuracy in DNA sequencing. According to CSE’s Bafna, until now, many applications were off-limits because current technologies were not accurate enough to be done at the level of the single human cell.
“Many individuals carry alleles that cause genetic disease or predispose them to cancer,” said Bafna. “Each gene has two alleles, one from each parent. One of the alleles may contain disease-causing mutations. The carriers may be asymptomatic, but their offspring may show symptoms due to the combination of bad alleles or haplotypes from both parents.”
Take the case of couples hoping to get pregnant via in-vitro fertilization (IVF). “For genetic diagnostics prior to IVF implantation, a human life is involved, so the utmost accuracy is required,” explained bioengineering professor Xiaohua Huang. “With our technology, we can do highly accurate sequencing and haplotyping of the genome based on a single cell biopsied from early embryos.”

three departments: (l-r): Vineet Bafna (CSE), Kun Zhang
and Xiaohua Huang (Bioengineering), and Vikas Bansal
(Pediatrics and faculty-affiliate in CSE).
In addition to IVF pre-implantation diagnostics and early cancer detection, other potential applications of the UC San Diego-developed technology include high-quality checking of genome-edited human cells for therapeutic purposes. “With the explosion in the use of CRISPR/Cas9 and other targeted genome-editing techniques, new treatments could be tweaked versions of the patient’s own cells,” said first author and bioengineering Ph.D. student Wai Keung Chu. “The technology makes it possible to use a single cell of the ‘edited’ gene and return results that would be as accurate as if we sequenced many cells.”

functional SISSOR device filled with dye solutions (red
for fluidic channels, blue for valves and valve lines);
(c) zoom-in view of regions with functional modules.
The technology in question has two novel aspects: a microfluidic processor that allows for the manipulation of single cells and separate chromosomal strands into different chambers; and computational methods that exploit the strand information for haplotyping and error correction. The UC San Diego scientists have dubbed it “Single-Stranded Sequencing using micrOfluidic Reactors” (SISSOR).
“Essentially it enables the simultaneous sequencing of very long fragments of all four strands of the chromosomal DNA from both parents,” explained CSE bioinformatics Ph.D. student Peter Edge, who works in Pediatrics professor Vikas Bansal’s Genome Information Science lab. “This allows us to make comparisons and correct for errors.”
The SISSOR technique also breaks what has been called the ‘curse of polymerases’, which introduce errors when making copies of DNA. Unfortunately, noted CSE’s Bafna, “we cannot read genome information from single cells without polymerases, so we had to come up with a solution that gets rid of those errors.”
Based on their findings, the PNAS paper’s co-authors were able to demonstrate “the most accurate single-cell genome sequencing to date.”

Suk Lee co-authored the
PNAS paper. Lee is an
ECE alumnus (M.S.,
Ph.D. ’11, ’15)
Senior author Kun Zhang says the interdisciplinary nature of the research collaboration was critical to finding a more precise way to sequence DNA from single cells. “Our approach can simultaneously provide higher accuracy and longer haplotypes than other existing approaches,” concluded Zhang. “This innovation required expertise that goes beyond what normally exists in a single department, and this case is a testament to UC San Diego’s growing interdisciplinary research culture that allowed us to pull in collaborators from other departments who were critical to a technology whose payoff will hopefully be measured in lives saved – and perhaps more healthy children born via in-vitro fertilization.”
________
*PNAS Paper: Ultra-accurate Genome Sequencing and Haplotyping of Single Human Cells, by Wai Keung Chu, Peter Edge, Ho Suk Lee, Vikas Bansal, Vineet Bafna, Xiaohua Huang and Kun Zhang, Proceedings of the National Academy of Sciences, October 24, 2017, doi:10.1073/pnas.1707609114

